18+ protons neutrons electrons calculator
Ferrum and atomic number 26. Alpha particles are a strongly ionizing form of radiation but when emitted by radioactive decay they have low penetration power and can be absorbed by a few centimeters of air or by the top.

Atomic Charge Calculator Calculator Academy
You get the idea.

. Sodium ion on right has 17 protons and 18 electrons with a -1 overall charge. Must contain at least 4 different symbols. Isotopes are the atoms in which.
In the case of a neutral atom a neutral atom contains an equal number of electrons and protons The number of electrons in a neutral atom is equal to the number of protons. There are 6 protons in accordance with the proton number in the subscript. The electrons have very less mass in comparison to protons or neutrons so the mass of electrons is not influenced in the calculation.
Each isotope of a given element has the same atomic number but a different mass number A which is the sum of the numbers of protons and neutrons. Though technically incorrect the term is also often used to refer to the average atomic mass of all of the isotopes of one element. 18 ka 370 ka 6000 1100 4000 K 04 eV Electrons and atomic nuclei first become bound to form neutral.
Draw the atomic structure of sodium atom. So it can vary from sample to sample. This is an alpha particle which can also be written as 4.
So a distinction is made between dose which is already in a location which is defined here as being background and the dose due to a deliberately. In physical cosmology Big Bang nucleosynthesis abbreviated BBN also known as primordial nucleosynthesis is the production of nuclei other than those of the lightest isotope of hydrogen hydrogen-1 1 H having a single proton as a nucleus during the early phases of the UniversePrimordial nucleosynthesis is believed by most cosmologists to have taken place in. The nucleus is made up of protons and neutrons and the electrons revolve around the nucleus.
AZX What does this symbol represent. The term is most commonly used in relation to atoms undergoing radioactive decay but can be used to describe other types of decay whether exponential or not. There are 6 electrons because the atom is neutral.
If a neutral atom has 2 protons it must have 2 electrons. How many electrons are present in the atom. In order to be neutral an atom must have.
The relative masses of atoms are reported using the atomic mass unit amu which is defined as one-twelfth of the mass of one atom of carbon-12 with 6 protons 6 neutrons and 6 electrons. The universe consists of a plasma of nuclei electrons and photons. Complete the following table.
The difference between the mass numberA and the atomic numberZ gives the number of neutrons n in a given nucleus. Charged Objects as an Imbalance of Protons and Electrons. 2 protons 2 neutrons 0 electrons.
Complete the following table. If a neutral atom has 1 proton it must have 1 electron. This measurement is the best indicator of aerobic endurance and cardiovascular fitness as it calculates how efficiently your cells use oxygen for energy.
Find the number of protons electrons an-d neutrons in a 13 27 A 3. 6 to 30 characters long. If a neutral atom has 10 protons it must have 10 electrons.
1 C 624 10 18 electrons. On the right the chloride ion has 18 electrons and has a 1 charge. His work was focused on electric forces between atoms and subatomic particles electrons protons neutrons etc.
In the previous section of Lesson 1 an atom was described as being a small and dense core of positively charged protons and neutral neutrons surrounded by shells of negatively charged electronsThe protons are tightly bound within the nucleus and not removable by ordinary measures. It tells what should be the total charge on a body if it has got n number of electrons or protons is calculated using Charge Number of Electron Charge-eTo calculate Electric Charge you need Number of Electron n electronWith our tool you need to enter the respective value for. In an element the number of protons is always the same but the number of neutrons keeps on changing.
It is the sum of the mass of electrons protons and neutrons present in an atom. This second definition is actually. 6 protons 8 neutrons 6 electrons.
On the left the chlorine atom has 17 electrons. Temperature is too low to create electron-positron pairs or any other pairs of massive particles but too high for the binding of electrons to nuclei. Background radiation is defined by the International Atomic Energy Agency as Dose or dose rate or an observed measure related to the dose or dose rate attributable to all sources other than the ones specified.
It is a metal that belongs to the first transition series and group 8 of the periodic tableIt is by mass the most common element on Earth right in front of oxygen 321 and 301 respectively forming much of Earths outer and inner coreIt is the fourth most common. Half-life is defined as the amount of time it takes a given quantity to decrease to half of its initial value. There are 8 neutrons because 14-68.
The periodic table of the chemical elements is a tabular method of displaying the chemical elements. Atomic mass is the sum of all the protons neutrons and electrons in a single atom or molecule. In order to be neutral an atom must have.
Iron ˈ aɪ ər n is a chemical element with symbol Fe from Latin. Name two elements with same number of protons and neutrons. Carbon Protons Neutrons 6 Oxygen Protons Neutrons 8 Question 10.
The Electric Charge magnitude value is always the integral multiple of the electric charge e. It is determined from the atomic number and the mass number. ASCII characters only characters found on a standard US keyboard.
The Formation of a Chlorine Ion. Simply add an amount to convert in the field above or select alternative units to work with. Name the isotope used for treatment of cancer.
The atomic weight depends on the relative abundance of isotopes of an element in a given sample. However the mass of an electron is so small it is considered negligible and not included in the calculation. 14 is the atomic mass number in the superscript.
For easy conversions between various charge units try our free nC to C calculator. If a neutral atom has 10 protons it must have 10 electrons. How many nucleons may be considered neutrons.
You get the idea. There are several methods you can use to measure VO 2 max but many require equipment such as a. Atomic mass is the sum of some protons and the number of neutrons and atomic number is equal to the number of protons.
If a neutral atom has 1 proton it must have 1 electron. Nucleons 254 electrons 102 and neutrons 254 102 152. If a neutral atom has 2 protons it must have 2 electrons.
N A Z. Alpha particles consist of two protons and two neutrons bound together into a particle identical to a helium nucleusAlpha particle emissions are generally produced in the process of alpha decay. Although precursors to this table exist its invention is generally credited to Russian chemist Dmitri Mendeleev in 1869.
Neutral chlorine atom on left has 17 protons and 17 electrons. An X-ray or much less commonly X-radiation is a penetrating form of high-energy electromagnetic radiationMost X-rays have a wavelength ranging from 10 picometers to 10 nanometers corresponding to frequencies in the range 30 petahertz to 30 exahertz 3 10 16 Hz to 3 10 19 Hz and energies in the range 145 eV to 124 keVX-ray wavelengths are shorter. The atomic mass of an isotopic atom is unique.
VO 2 max is a measure of the maximum amount of oxygen that you use during intense physical activity. For an element relative atomic mass is the average mass of the naturally occurring isotopes of that element relative to the mass of an atom of 12C.

Odzvsibi736k0m
Average Atomic Mass Calculator
Atomic Structure
Protons Ions Niel Calculator
Atomic Mass Calculator Find Average Definition Formula

7 Best Free Atomic Mass Calculator Online Websites

Atom Calculator On The App Store

Atomic Mass Calculator Calculate Neutrons Protons Electrons
Gauss S Law Calculator Calculate The Electric Flux

How To Find The Number Of Protons Neutrons And Electrons

Atom Calculator By Gorasiya Vishal Nanjibhai
Force Calculator Formula Calculator Academy
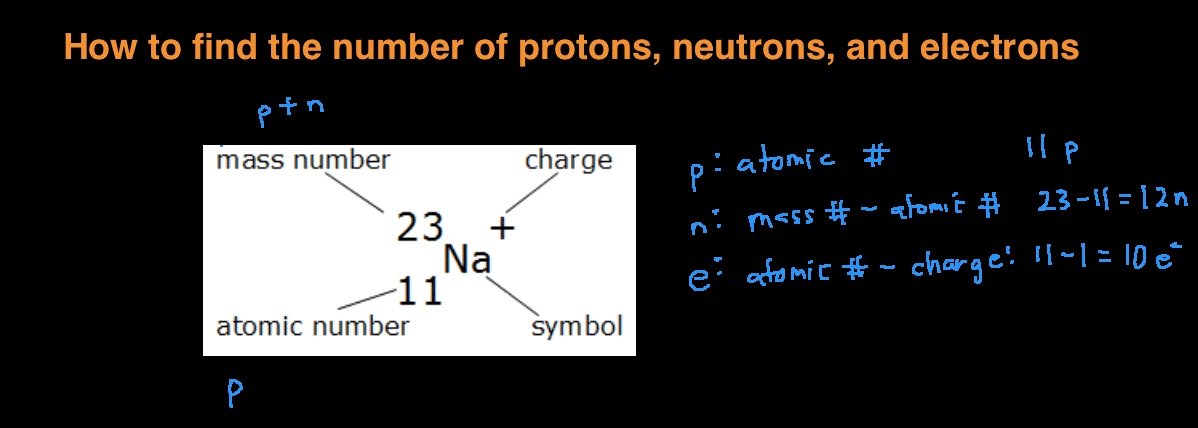
How To Find The Number Of Protons Neutrons And Electrons
Atomic Mass Formula Definition Calculating Atomic Mass Problems

7 Best Free Atomic Mass Calculator Online Websites
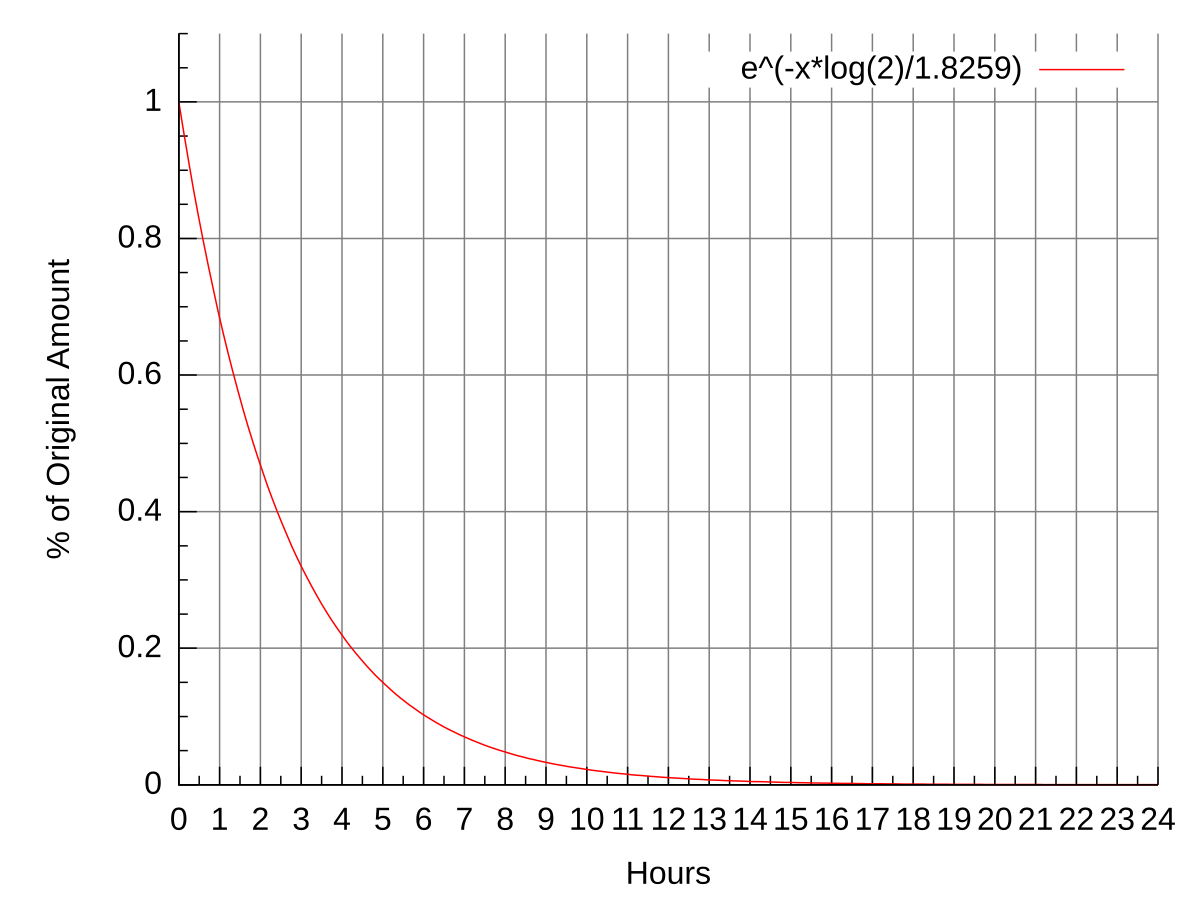
Fluorine 18 Wikipedia
Does A Calculator Display Screen Emit Any Radiation Quora